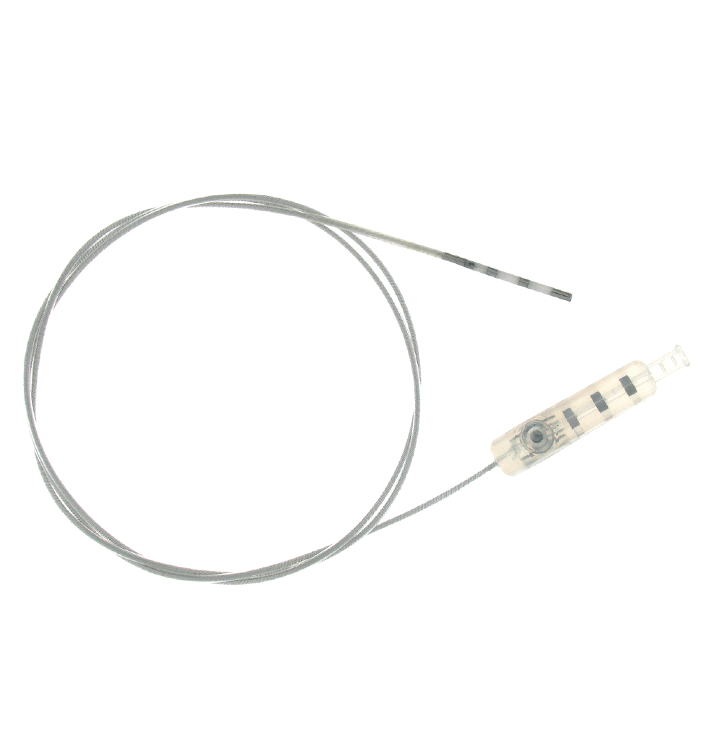
Neurimpulse’s mission is improve patient’s quality of life suffering from chronic pain trough the permanent implantable neuromodulation.
Our target is the treatment of pain related to peripheral neuropathies.

Our aim is to improve patient’s quality of life suffering from chronic pain.
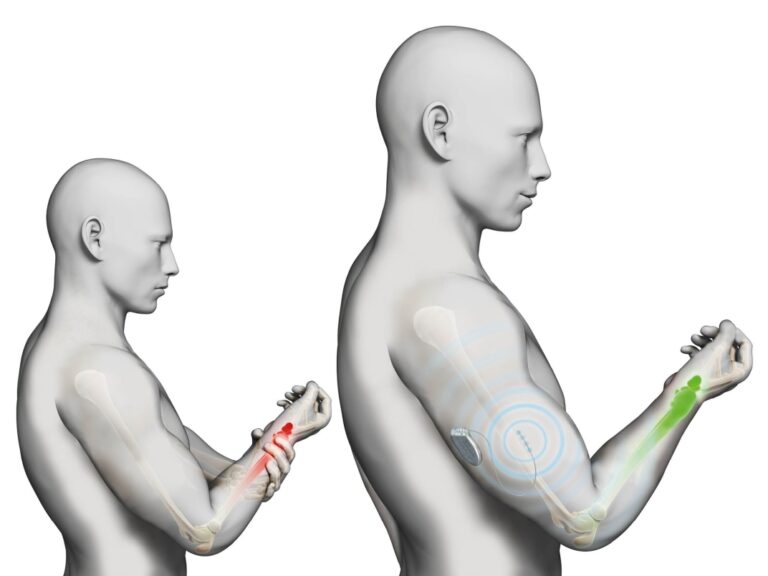
Neurimpulse system has been studied for many years to treat peripheral neuropathies such as craniofacial neuralgia, phantom limb syndromes, type II complex regional pain syndromes, post-traumatic pain and post-surgical pain.
Clinical studies show excellent benefits following the use of peripheral neuromodulation therapy.
PAIN REDUCTION
Trated patients
80% of treated patients confirm the benefit during the trial phase
publications relating to clinical studies on the system
The first CE mark was released by the notified body 0373 in 2009 for Lightline leads family.
Subsequently, in 2010, the CE mark was also obtained for the implantable stimulator, temporary stimulator, programmer for the doctor and programmer for the patient in addition to the various leads, extensions and accessories.
Currently, all neurostimulation products have CE mark, confirming that Research and Development, Production, Post-Marketing, Quality Assurance and the other departments that interact in the internal process carry out their activities in accordance with the dispositions established by European Union, in particular the directive 90/385/EEC and subsequent changes and additions.
Furthermore, the company is certified according to the international standard ISO13485:2016.